Many diseases and disorders studied at Van Andel Institute have genetic links, including cancer and Parkinson’s disease. Gene mutations, or changes to genes, play a major role in disease. Here’s a quick primer.
What are genes?
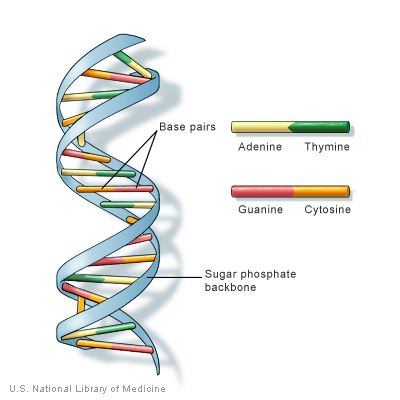
Before we get too deep, let’s cover the basics. Most human cells have 23 pairs of chromosomes, for a total of 46 individual chromosomes. We inherit our chromosomes from our parents — one from our mother, and one from our father. Each chromosome consists of tightly coiled DNA (deoxyribonucleic acid), a ladder-like molecule that carries all of the instructions needed for life. The “rungs” of DNA’s ladder comprise pairs of four chemicals — adenine and thymine, and cytosine and guanine. Different patterns of these chemical pairs result in instructions for making everything your body needs for you to be you.
Genes are segments of DNA that determine specific human characteristics such as height, eye color or the risk of developing a certain disease. Most genes are the same in all people, but a small number of them (less than 1% of the total) differ slightly between individuals. These alleles — the forms of the same genes with small differences in the sequence of their DNA bases, or the chemical “rungs” of the ladder — contribute to each person’s unique features.
Some characteristics arise from a single gene, while others result from gene combinations. The Human Genome Project estimated that humans have between 20,000 and 25,000 genes, and every person has two copies of each gene — one inherited from each biological parent.
Epigenetics, or shifts in how our genetics are regulated, play a role in our understanding of genes and their expression, too. Read more about it here.
What is a gene mutation?
A gene mutation is a permanent change in the DNA sequence that makes up a gene. When this occurs, we’re left with a sequence that differs from what is found in most people. Mutations can affect a single DNA base pair to a large segment of a chromosome that includes multiple genes.
Gene mutations can be classified in two major ways:
- Hereditary mutations are inherited from a parent and are present throughout a person’s life in virtually every cell in the body. Also known as germline mutations, these changes come from the parent’s egg or sperm cells; when these germ cells unite, the resulting fertilized egg cell gets its DNA from both parents. If this DNA has a mutation, the offspring will have the mutation in each of their cells.
- Acquired mutations are the opposite. They occur after the fertilization of an egg cell and can be caused by environmental factors such as ultraviolet radiation from the sun or errors made as DNA copies itself during cell division. These are also the most common cause of cancer (germline mutations account for only about 5% to 20% of all cancers).
Acquired mutations in cells other than sperm and egg cells cannot be passed to the next generation and are known as somatic mutations.
Are all mutations harmful?
No, not all mutations are harmful. Most disease-causing gene mutations are relatively uncommon in the general population.
Take for example polymorphisms, which are variations of a particular genetic sequence. Common enough to be considered a normal variation in DNA, polymorphisms result in many of the normal differences between people such as eye color, hair color and blood type. Some polymorphisms involve a single change at a base pair (DNA’s “rungs”) while others are much bigger.
Though many polymorphisms don’t negatively affect a person’s health, some variations may influence the risk of developing certain disorders and can serve as biological markers that may help scientists locate genes associated with specific diseases.
How can we use our understanding of gene mutations to battle disease?
When we know what a specific gene or a mutation does, we can identify when something has gone awry and work to develop ways to fix the problem. Finding certain mutations in cells helps confirm diagnoses of specific cancers, and can be used after diagnosis to determine the therapies to which the cancer will best respond.
By understanding the genetic components and gene mutations associated with specific diseases, VAI scientists can translate their discoveries into actionable prevention and treatment techniques to help people live longer, healthier lives. A few recent examples include:
- The work being done in the Moore Laboratory to uncover the genetic causes of Parkinson’s disease, with the goal of developing targeted therapies for the disease.
- Research related to genome-wide association studies (GWAS) conducted in VAI’s Coetzee Laboratory with the goal of understanding how the genetic predisposition of complex diseases such as cancer and Parkinson’s impose risk to aid in the development of therapies that slow or halt the diseases.
Work related to The Cancer Genome Atlas (TCGA), a landmark project that revolutionized the understanding of cancer and that is hailed as an exemplar of scientific collaboration. The more than decade-long initiative, led by the National Institutes of Health, was the most in-depth undertaking of its kind, and included analysis of 10,000 tumors across 33 cancer types.
