New experimental blood test determines which pancreatic cancers will respond to treatment
October 21, 2020
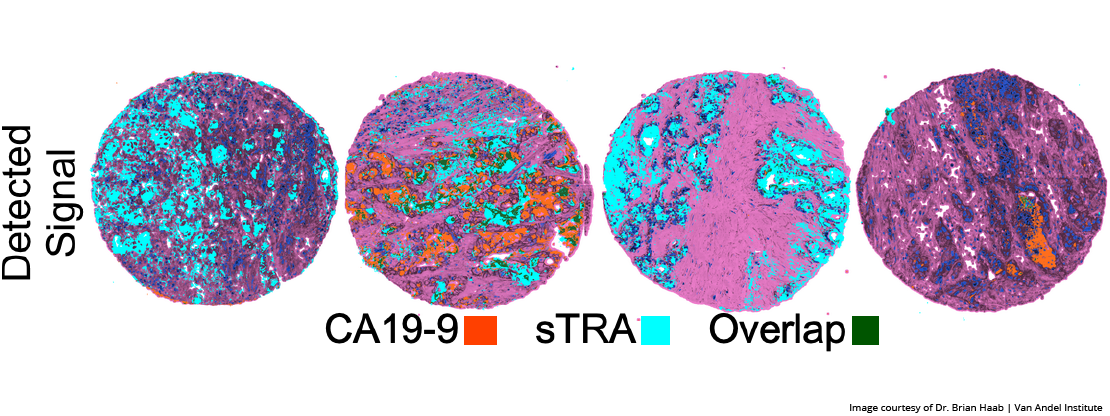
GRAND RAPIDS, Mich. (Oct. 22, 2020) — Scientists have developed a simple, experimental blood test that distinguishes pancreatic cancers that respond to treatment from those that do not. This critical distinction could one day guide therapeutic decisions and spare patients with resistant cancers from undergoing unnecessary treatments with challenging side effects.
The findings were published today in Clinical Cancer Research, a journal of the American Association for Cancer Research.

“Knowing which type of pancreatic cancer a person has is critical to implementing the right treatment strategy for each patient,” said Brian Haab, Ph.D., a professor at Van Andel Institute and corresponding author of the study. “We hope that our new test, which detects a marker produced by cancer cells of one subtype and not the other, will one day soon be a powerful tool to help physicians and patients make the best decisions possible.”
Pancreatic cancers are among the most challenging malignancies to treat, due in part to their ability to evade detection until they have advanced and spread. Physicians currently have no reliable way to determine whether a patient has a subtype that will respond to existing chemotherapies versus a subtype that is resistant to treatment. The result often is a blanket treatment approach that works in only some but can have side effects in all.
The test detects and measures the levels of a sugar called sTRA, which is produced by a certain subtype of pancreatic cancer and escapes into the blood stream. Pancreatic cancers that produce sTRA tend to not respond to chemotherapy.
The new sTRA test evolved from an earlier test announced in January 2019. In that study, also published in Clinical Cancer Research, Haab and his colleagues described an experimental blood test that combined an existing diagnostic that detected a sugar called CA19-9 with a new test that detected sTRA. The combination approach detected nearly 70% of pancreatic cancers with a less than 5% false-positive rate — roughly 30% more than the CA19-9 alone. Both the 2019 combination test and the new sTRA test are slated to undergo additional clinical validation.
“The 2019 combination test tells us whether there is cancer and the new sTRA test helps us determine what kind of pancreatic cancer, which then could allow physicians to better narrow down the appropriate treatment plan,” Haab said. “When used in sequence, we believe the combination test and the new sTRA test could help catch and identify pancreatic cancer more quickly and definitively.”
Authors include ChongFeng Gao, Ph.D., Luke Wisniewski, Ying Liu, Ph.D., Ben Staal, Ian Beddows, Ph.D., Johnathan Hall and Daniel Barnett, Ph.D., of VAI; Dennis Plenker, Ph.D., Mirna Kheir Gouda and David A. Tuveson, M.D., Ph.D., of Cold Spring Harbor Laboratory; Mohammed Aldakkak, M.D., Douglas Evans, M.D., FACS, and Susan Tsai, M.D., MHS, of Medical College of Wisconsin; Peter Allen, M.D., of Duke University School of Medicine; Richard Drake, Ph.D., of Medical University of South Carolina; Amer Zureikat, M.D., Aatur Singhi, M.D., Ph.D., and Randall E. Brand, M.D., of University of Pittsburgh Medical Center; and Ying Huang, Ph.D., of Fred Hutchinson Cancer Research Center. VAI’s Optical Imaging Core, Bioinformatics and Biostatistics Core, Genomics Core and Pathology and Biorepository Core also supported this work.
Research reported in this publication was supported by Van Andel Institute; the National Cancer Institute of the National Institutes of Health under award no. U01CA152653 (Haab and Brand) and award no. U01CA226158 (Haab); the Lustgarten Foundation (Tuveson); and the German Research Foundation (Plenker). The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting organizations.
###
ABOUT VAN ANDEL INSTITUTE
Van Andel Institute (VAI) is committed to improving the health and enhancing the lives of current and future generations through cutting edge biomedical research and innovative educational offerings. Established in Grand Rapids, Michigan, in 1996 by the Van Andel family, VAI is now home to more than 400 scientists, educators and support staff, who work with a growing number of national and international collaborators to foster discovery. The Institute’s scientists study the origins of cancer, Parkinson’s and other diseases and translate their findings into breakthrough prevention and treatment strategies. Our educators develop inquiry-based approaches for K-12 education to help students and teachers prepare the next generation of problem-solvers, while our Graduate School offers a rigorous, research-intensive Ph.D. program in molecular and cellular biology. Learn more at vai.org.
Media Contact
Beth Hinshaw Hall
Van Andel Institute
[email protected]
616-822-2064
