Switching off ‘master regulator’ may shield the brain from Parkinson’s-related damage
August 16, 2020
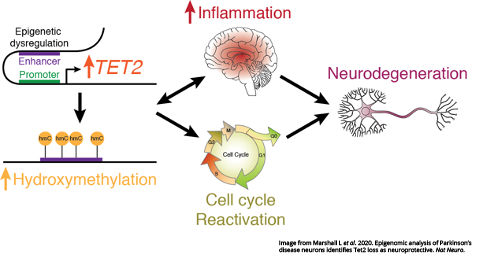
GRAND RAPIDS, Mich. (August 17, 2020) — Switching off a molecular “master regulator” may protect the brain from inflammatory damage and neurodegeneration in Parkinson’s disease, reports a study published today in Nature Neuroscience.
The study is the first of its kind and points to an entirely new avenue for developing therapies that preserve vulnerable brain cells in Parkinson’s disease. Currently, there are no effective ways to prevent Parkinson’s or to slow or stop its progression.
“One of the biggest challenges in treating Parkinson’s, other than the lack of therapies that impede disease progression, is that the disease has already laid waste to significant portions of the brain by the time it is diagnosed,” said Viviane Labrie, Ph.D., an associate professor at Van Andel Institute and the study’s senior author. “If we can find a way to protect critical brain cells from Parkinson’s-related damage early on, we could potentially delay or even prevent symptom onset.”

Labrie and her colleagues analyzed the brains of people with Parkinson’s alongside healthy controls. They found TET2 was overactive in Parkinson’s disease and that epigenetic dysfunction linked to altered TET2 affected genes involved in the reactivation of the cell cycle and a heightened immune response. While restarting the cell cycle is normal for other cells types, it is fatal to neurons.
At the same time, their findings in the mouse brain showed reducing Tet2 activity protects neurons from inflammatory insults and the subsequent neurodegeneration that is a hallmark of Parkinson’s. Using a model of infection that results in a loss of dopamine neurons relevant to Parkinson’s, the team also found that Tet2 inactivation suppressed pro-inflammatory gene activity, brain immune cell activation and the eventual death of neurons triggered by inflammation.
Taken together, their findings suggest that calming TET2 activity may one day be a powerful preventative measure. In the future, such a strategy could be employed after a person experiences a major inflammatory event, such as an infection, to alleviate residual inflammation without interfering with its normal, healthy role in the body.
“Parkinson’s is a complex disease with a range of triggers. Temporarily reducing TET2 activity could be one way to interfere with multiple contributors to the disease, especially inflammatory events, and protect the brain from loss of dopamine-producing cells” Labrie said. “More work is needed before a TET2-based intervention can be developed, but that it is a new and a promising avenue that we already are exploring.”
Authors include Lee Marshall, Ph.D., Elizabeth Ensink, Peipei Li, Ph.D., Katie Li, Wei Cui, Ph.D., Noah Lubben, Xinhe Wang, Juozas Gordevicius, Ph.D., and Gerhard A. Coetzee, Ph.D., of VAI; Matthew Weiland and Stefan Jovinge, M.D., Ph.D., of the DeVos Cardiovascular Research Program, a joint effort between VAI and Spectrum Health; Bryan Killinger, Ph.D., of Rush Medical College; and Jiyan Ma, Ph.D., formerly of VAI. The VAI Flow Cytometry Core, Genomics Core, Bioinformatics and Biostatistics Core, Pathology and Biorepository Core and Vivarium and Transgenic Core; and the CAHM Sequencing Facility supported this work. Brain tissue was provided by the Parkinson’s UK Brain Bank, the NIH NeuroBioBank and the Michigan Brain Bank.
The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Congressionally Directed Medical Research Programs’ Parkinson’s Research Program under Award No. W81XWH1810512 (Labrie). Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. In conducting research using animals, the investigators adhere to the laws of the United States and regulations of the Department of Agriculture. Animal research at VAI is conducted in accordance with the National Institutes of Health’s Office of Laboratory Animal Welfare Public Health Service Policy on Humane Care and Use of Laboratory Animals. VAI also is accredited by AAALAC International.
This work also was supported by a VAI Innovation Award (Labrie).
Labrie also has awards from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health and Michigan State University through the Gibby & Friends vs. Parky Parkinson’s Disease Research Award.
###
ABOUT VAN ANDEL INSTITUTE
Van Andel Institute (VAI) is committed to improving the health and enhancing the lives of current and future generations through cutting edge biomedical research and innovative educational offerings. Established in Grand Rapids, Michigan, in 1996 by the Van Andel family, VAI is now home to more than 400 scientists, educators and support staff, who work with a growing number of national and international collaborators to foster discovery. The Institute’s scientists study the origins of cancer, Parkinson’s and other diseases and translate their findings into breakthrough prevention and treatment strategies. Our educators develop inquiry-based approaches for K-12 education to help students and teachers prepare the next generation of problem-solvers, while our Graduate School offers a rigorous, research-intensive Ph.D. program in molecular and cellular biology. Learn more at vai.org.
