Van Andel Institute, University of Freiburg study reveals insights into enzyme that combats a common greenhouse gas
July 27, 2022
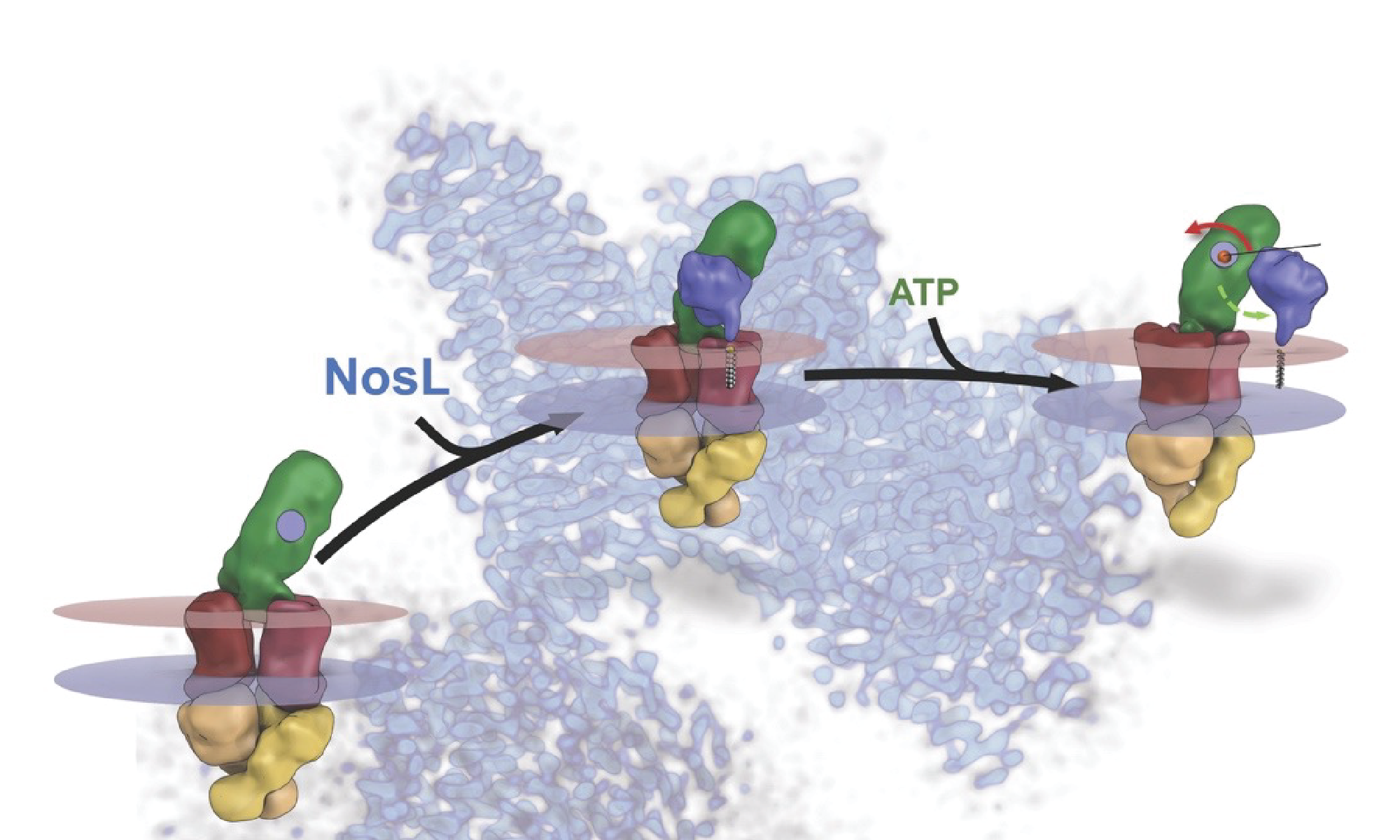
GRAND RAPIDS, Mich. (July 27, 2022) — An enzyme that combats the greenhouse gas nitrous oxide (N2O) may one day give scientists a potent new tool for reducing the amount of the gas in the atmosphere thanks in part to new findings published today in Nature.

The study details how the enzyme — N2O reductase — is assembled and offers key insights into its ability to render nitrous oxide into harmless nitrogen and water. The research was co-led by VAI Associate Professor Juan Du, Ph.D., VAI Associate Professor Wei Lü, Ph.D., and University of Freiburg Professor Oliver Einsle, Ph.D.
“Addressing greenhouse gases is a massive, multi-faceted endeavor. Today’s findings are an early but important step toward development of another tool to potentially combat one contributor to climate change,” Du said.

Greenhouse gases trap heat in the Earth’s atmosphere and contribute to increasing global temperatures. Nitrous oxide only accounts for about 7% of greenhouse gases produced by human activities but its impact is 300-fold that of the most common greenhouse gas, carbon dioxide. Nitrous oxide is most frequently generated by agricultural practices, such as the use of nitrogen fertilizers, according to the U.S. Environmental Protection Agency. It can remain in the atmosphere for more than a century.
N2O reductase is used by certain microbes to break down nitrogen-based molecules as part of the Earth’s natural nitrogen cycle. Use of nitrogen-heavy fertilizers can overwhelm these microbes’ ability to fully mitigate nitrous oxide, allowing it to escape into the atmosphere. Understanding exactly how this happens is a crucial step toward strategies to mediate nitrous oxide, thus reducing atmospheric levels.
Regarding the biotechnological applications of N2O reductase, it is crucial to understand and control the supply of copper ions during the assembly of the enzyme in the cell. The study centered on the structure of a membrane protein complex called NosDFY and the way it accomplishes N2O reductase assembly. Using a host of mapping and modeling techniques, the team discovered that NosDFY acts as a conduit that converts chemical energy into mechanical energy, which in turn powers the delivery of copper ions required for the creation of more N2O reductase.
The findings reshape a decade-old belief about this crucial copper delivery system and reveal a novel mode of operation for similar molecules. Although additional research is needed, the findings provide a detailed blueprint that may be translated into future environmental remediation strategies.
Christoph Müller and Lin Zhang of the Institute of Biochemistry at University of Freiburg are co-first authors of the study. Other authors include Sara Zipfel, Annika Topitsch, Marleen Lutz, Johannes Eckert and Benedikt Prasser of the Institute of Biochemistry at University of Freiburg; and Mohamed Chami of the BioEM Lab at University of Basel. The following groups also contributed to this project: VAI’s David Van Andel Advanced Cryo-Electron Microscopy Suite, the Pacific Northwest Center for Cryo-EM, VAI’s high-performance computing team, and the bwHPC Cluster.
Research reported in this publication was supported by European Research Council under award no. 310656 (Einsle); Deutsche Forschungsgemeinschaft under award no. CRC1381 (project ID: 403222702; Einsle) and award no. RTG2202 (project ID: 46710898, Einsle); the BIOSS Centre for Biological Signaling Studies at Albert-Ludwigs-Universität Freiburg (Einsle); a McKnight Scholar Award (Du); a Klingenstein-Simons Fellowship Award in Neuroscience (Du); a Sloan Research Fellowship in Neuroscience (Du); a Pew Scholars in Biomedical Research Award from the Pew Charitable Trusts (Du); and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award no. R01NS111031 (Du).
