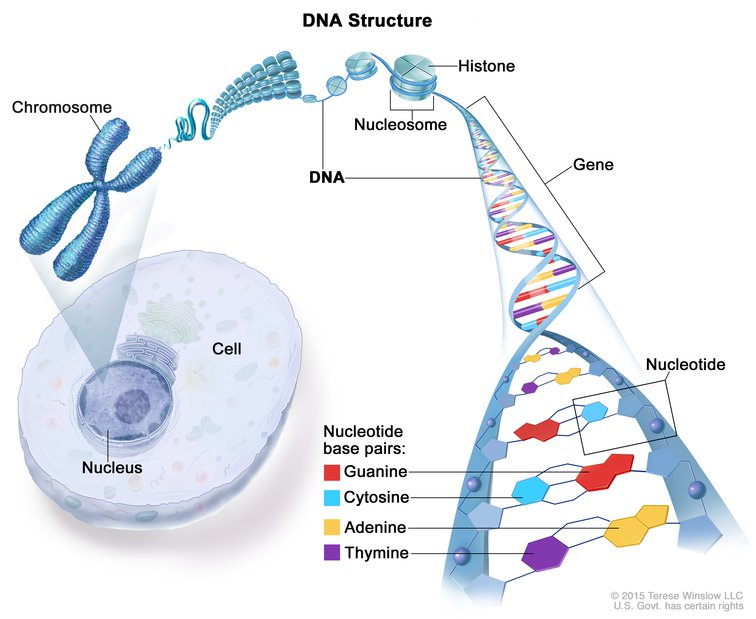
KEY TERMS
Chromosomes: The X-shaped structure of a chromosome consists largely of tightly coiled DNA, which contains the genetic instruction manual. Without the packaging provided by chromosomes, DNA strands would be far too long to fit inside a cell. Humans have a total of 46 chromosomes that are divided into 23 pairs. Learn more here.
Chromosomal instability: Chromosomal instability (CIN) is a common feature in many cancers. Put simply, it means that something is wrong with the chromosomes—parts may be missing or changed—which then causes the host cell to either die or become cancerous, in some cases.
Genes: Genes are stretches of DNA that contain instructions for a specific biological task or trait. Whether the gene is active or not is controlled by a host of factors through a process called gene regulation. Learn more here.
Metastasis: Metastasis occurs when cancer spreads from its original site in the body to another site, such as prostate cancer spreading to the bones. For a cancer to be metastatic, it must be proliferative (meaning the cells reproduce uncontrollably) and invasive (meaning it actively moves to and colonizes other tissues).
Epithelial cells: Specialized cells that adhere tightly to one another, forming sheets of cells that line body cavities and organs.
Mesenchymal cells: Undifferentiated cells that can become other types of cells, such as bone or muscle.
The short version
Scientists have uncovered key clues to a longstanding mystery—why do some cancers progress and spread, or metastasize, while others don’t? The answer lies in instability of the chromosomes, the X-shaped, thread-like structures that hold the genetic code.
The new findings suggest that chromosomes in tumor cells are constantly changing, gaining and losing pieces that eventually make the cells less specialized, allowing them to more easily spread and take root elsewhere in the body. The instability also leads to the less specialized cells to produce more specialized variants that are suited to specific environments, allowing them to proliferate and colonize new areas.
Why are these findings important?
Once a cancer metastasizes, it becomes much more difficult to treat. A better understanding of how and why this process occurs give scientists crucial new tools for developing strategies that interfere with or even prevent it.
The science
Chromosomal instability is a key feature of many cancers yet its role in driving disease progression to the point of metastasis was largely unknown. Using human cancer cell lines, Senior Research Scientist Dr. ChongFeng Gao and Vande Woude Laboratory team showed that epithelial cells were capable of generating mesenchymal variants; this is critical in cancer because mesenchymal cells are more invasive, and can revert back to more proliferative epithelial cells.
The switch from epithelial to mesenchymal and back again is driven by changes in chromosome content, specifically genes linked to metastasis and genes that regulate intercellular junctions, structures that connect neighboring cells to each other.
Here’s how it works: Switching off the genes that regulate junctions (appropriately called intercellular junction genes) facilitates the production of mesenchymal cells from epithelial cells. On the reverse side, switching off a gene called ZEB1 in mesenchymal cells leads to the generation of epithelial cells. Taken together, the whole process promotes the spread of cancer and its ability to dig in at other areas of the body. These results suggest that both the epithelial and the mesenchymal cells need to be targeted therapeutically.
Scientist’s take:
“It’s important to remember that metastatic cancers must have two specific characteristics—they must be proliferative and they must be invasive. It’s not enough to have one or the other,” said George Vande Woude, Ph.D., Distinguished Scientific Fellow at VARI. “Understanding this process gives us crucial insight into a very complex and variable group of diseases. It is our hope that our results aid in the development of new therapies that better impede cancer’s ability to spread.”
Where can I read more?
The full scientific paper is available from the Proceedings of the National Academy of Sciences here.
Gao CF, Su Y, Koeman J, Haak E, Dykema K, Essenburg C, Hudson E, Petillo D, Khoo SK, Vande Woude GF. 2016. Chromosome instability drives phenotypic switching to metastasis. Proc Natl Acad Sci U S A. 14793–14798.