High-resolution “blueprint” reveals mechanism that helps E. coli infect the urinary tract
The findings may aid in the development of precision antimicrobial therapies
October 2, 2018

GRAND RAPIDS, Michigan (October 3, 2018) — A common gut bacterium uses a unique assembly apparatus to build hair-like structures that help it infect the bladder and kidneys. The findings, published today in Nature, depict how these structures — called pili — are produced, offering new avenues for the development of targeted antimicrobial medications for urinary tract infections.
“E. coli bacteria are the predominant cause of urinary tract infections, which affect more than 150 million people around the world annually,” said Huilin Li, Ph.D., a professor at Van Andel Research Institute (VARI) and a senior author of the study. “These infections are often treated with broad-spectrum antibiotics that, despite their importance as a medical tool, are increasingly problematic due to their potential to cause drug resistance and their tendency to disrupt the body’s microbial balance. More precise anti-microbial therapies that target specific bacteria but spare others are desperately needed.”
E. coli belong to a large, diverse family of bacteria that reside in the guts of humans and animals, where they are a normal part of the digestive tract’s complex ecosystem. Although most strains of E. coli are not harmful to the gut, they can cause painful infections elsewhere in the body, such as in the urinary tract. In severe cases, the bacteria travel to the kidneys where they can cause a life-threatening condition called acute pyelonephritis, which is marked by swelling, high fever, pain and blood in the urine.
Once in the urinary tract, E. coli use hair-like structures called Type 1 pili to latch on to host cells, allowing them — and the infection they cause — to take root. Construction of the pili is a complicated, multi-step process that, until now, has not been clearly delineated.
Using a technique called cryo-EM, which makes it possible to image molecular architecture at the atomic level, Li and collaborator David G. Thanassi, Ph.D., of Stony Brook University, visualized a protein complex that allows pili assemblage to occur, enhancing the understanding of E. coli-related urinary tract infections and providing a possible drug target.
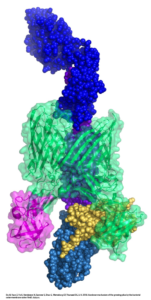
“FimD usher is exceptionally dynamic. It performs a series of complicated tasks to assemble a pilus — it uses one part of its structure to recruit distinct proteins in an orderly manner, catalyzes subunit polymerization and then hands over the recruited pilus proteins to another part of the structure for secretion to the cell surface. While the structures involved in some of these steps have been determined by others, this study is the first to show how the handover step is accomplished,” Li said. “Surprisingly, the mechanism involves a meeting of two ends of FimD usher, which in a way resembles an ouroboros, the mythical snake eating its own tail.”
In the U.S. alone, urinary tract infections result in more than 10 million visits to the doctor’s office and 2 to 3 million trips to the emergency room each year, with an economic burden of more than $3.5 billion annually, according to a 2015 report in Nature Reviews Microbiology. Women and girls have a higher risk of developing urinary tract infections; in fact, more than half the women in the world have reported having a urinary tract infection at some point in their lives. Older populations and children also are at increased risk.

Authors include Minge Du, a graduate student at Van Andel Institute Graduate School and the study’s first author, Zuanning Yan, Ph.D., and Hongjun Yu, Ph.D., both members of Li’s laboratory at VARI; Gongpu Zhao, Ph.D., of VARI’s David Van Andel Advanced Cryo-Electron Microscopy Suite; and Nadine Henderson, Samema Sarowar, Ph.D., and Glenn T. Werneburg, M.D, Ph.D., of Stony Brook University.
Research reported in this publication was supported by the National Institutes of Health under grant number GM062987 (Thanassi) and Van Andel Research Institute (Li). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
###
ABOUT VAN ANDEL RESEARCH INSTITUTE
Van Andel Institute (VAI) is an independent nonprofit biomedical research and science education organization committed to improving the health and enhancing the lives of current and future generations. Established by Jay and Betty Van Andel in 1996 in Grand Rapids, Michigan, VAI has grown into a premier research and educational institution that supports the work of more than 400 scientists, educators and staff. Van Andel Research Institute (VARI), VAI’s research division, is dedicated to determining the epigenetic, genetic, molecular and cellular origins of cancer, Parkinson’s and other diseases and translating those findings into effective therapies. The Institute’s scientists work in onsite laboratories and participate in collaborative partnerships that span the globe. Learn more about Van Andel Research Institute or donate by visiting vari.vai.org. 100% To Research, Discovery & Hope®