Explainer: How genetics impact Alzheimer’s, Parkinson’s and Lewy body dementia
April 21, 2019
On May 7, the Institute will host A Focus on Parkinson’s, Alzheimer’s and Lewy Body Dementia, the next installment in our Public Lecture Series. Scientists Dr. Rita Guerreiro and Dr. José Brás will explain the role genetics plays in these devastating diseases and explain how new findings could point the way to improved treatments. The event is free and open to the public. Learn more and register here.
 ALZHEIMER’S DISEASE
ALZHEIMER’S DISEASE
Stats: 44 million people worldwide | 5.5 million in the U.S.
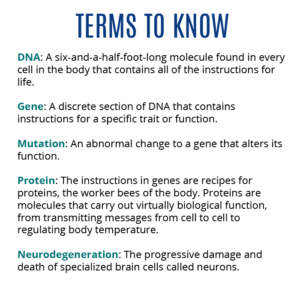
Disease profile: Alzheimer’s disease is the most common neurodegenerative disorder in the world and a leading cause of death among older Americans. It is marked by progressive loss of memory and cognitive abilities such as thinking, reasoning or performing simple tasks. Evidence suggests that the earliest stages of Alzheimer’s may start a decade or earlier before symptoms begin. Although a person may not show outward signs during the time, changes in the brain — namely the tangling of proteins called tau and the buildup of amyloid plaques — are occurring and causing the damage that leads to symptoms.
Read more at the National Institutes of Health website.
How are genetics involved? Genetic variants are important risk factors for Alzheimer’s although other influences, such as lifestyle issues (smoking, excessive alcohol consumption) and health problems (diabetes and hypertension), also may be at play. One prominent idea is called the amyloid cascade hypothesis, which suggests that mutations to three genes — APP, PSEN1 and PSEN2 — lead to the build-up of beta-amyloid, exceptionally “sticky” protein fragments that eventually form plaques. As these plaques accumulate, they begin to interfere with communication between cells, which inappropriately may activate the immune system. This then leads to chronic inflammation, which damages and kills brain cells, triggering the disease’s symptoms.
There are other important genes as well, including:
- APOE: Having certain types of the APOE gene can increase a person’s risk of developing Alzheimer’s.
- TREM2: TREM2 has recently been found to cause a similar type of dementia to Alzheimer’s and is being investigated as a genetic risk for Alzheimer’s disease. It is the most significant genetic risk factor identified since the discovery of APOE.
PARKINSON’S DISEASE
Stats: 7 to 10 million people worldwide | 500,000 people in the U.S.
Disease profile: Parkinson’s disease is the second most common neurodegenerative disorder and, for a long time, was considered to be strictly movement related. In recent years, however, it has become clear that Parkinson’s has a host of movement and non-related movement symptoms, including tremor, the progressive loss of voluntary movement, “freezing,” gastrointestinal issues, depression, loss of sense of smell and cognitive decline. Like Alzheimer’s, it too is believed to begin years before symptoms are first noticed and is marked by the buildup of toxic proteins in cells, specifically abnormally shaped alpha-synuclein. These proteins stick together like grains of rice, forming clumps called Lewy bodies that many scientists believe clog up normal cellular machinery, eventually damaging and killing brain cells responsible for day-to-day function.
How are genetics involved? More than 90 percent of Parkinson’s cases are sporadic, meaning that we don’t know exactly what gives rise to the disease. Growing evidence suggests that it is caused by a combination of factors that vary from person to person, including genetics, shifts in how our genetics are regulated (a set of processes called epigenetics) and environmental exposures. These changes slowly accumulate until they reach a critical point, eventually tipping the scale toward Parkinson’s.
In less than 10 percent of cases, Parkinson’s can be directly linked to a change in specific genes that have been passed down through families (although it is important to note that having a mutation in one of these genes does not always mean a person will get Parkinson’s).
Some of the main genes implicated in Parkinson’s disease include:
LRRK2: LRRK2 mutations are found in an estimated 2 percent of all people with Parkinson’s.
GBA: The GBA gene is an important regulator of cellular waste removal systems. Problems with GBA can cause these systems to break down, allowing molecular debris to build up and damage cells. It is one of the most frequently mutated genes across the Parkinson’s spectrum, including sporadic and familial cases.
SNCA: SNCA contains instructions for the protein alpha-synuclein, abnormal forms of which comprise Lewy bodies, clumps of alpha-synuclein that are a hallmark of Parkinson’s. Rare DNA changes in SNCA can cause Parkinson’s, while more common changes can modify the risk for a person to develop the disease.
PRKN: PRKN is one of the biggest genes in humans. The protein for which it codes is called parkin, which supports waste removal in cells by marking debris with chemical tags. These tags are recognized by special cellular waste collectors and are disposed of.
Learn more about genetics and Parkinson’s in our recent blog post here.
LEWY BODY DEMENTIA
Stats: More than 1.3 million in the U.S.
*Because it is understudied, the incidence of Lewy body dementia is difficult to pin down. Current numbers are believed to be low due to difficulty diagnosing the disease.
Disease profile: Lewy body dementia is the second most common type of dementia after Alzheimer’s, and is particularly difficult to diagnose because it shares many of the same features and symptoms of Parkinson’s and Alzheimer’s diseases. Like Parkinson’s, it is marked by the presence of clumps of abnormal alpha-synuclein, or Lewy bodies, and counts loss of movement among its symptoms. And, like Alzheimer’s, it causes deterioration of memory and cognitive function. These similarities mean it is frequently misdiagnosed, especially in its early stages, which can lead to significant problems for patients (many Alzheimer’s medications, for example, can have adverse effects on people with Lewy body dementia).
Lewy body dementia also typically causes a significant decline in mental abilities, such as judgement, and frequently causes sleep disturbances. It can be split into two main subtypes — dementia with Lewy bodies (which affects cognitive parts of the brain first) and Parkinson’s disease dementia (which affects movement before cognition).
Read more at the National Institutes of Health website.
How are genetics involved?
Gene variants implicated in Alzheimer’s and Parkinson’s diseases have also been linked to Lewy body dementia, including APOE, GBA and SNCA, but the exact cause of the disease remains largely unknown. However, scientists are hard at work investigating how changes to genes and to the parts of DNA that regulate genes may give rise to the disease or increase risk. Recently, Drs. Brás and Guerreiro and colleagues scoured the total sets of genetic material from 2,000 people with Lewy body dementia and 5,000 people without the disease to see if they could identify how much of a role genetics play. Their results? Lewy body dementia has a clear, measurable and significant genetic component that differentiates it from Parkinson’s and Alzheimer’s.
How can understanding genetic risk in these diseases help?
Genetic mutations are prime targets for new medications, which can be designed to “fix” genetic errors and get cells working properly again. To do this, however, scientists need to know which genes are involved in the disease, how mutations to these genes impact disease and which type of drug will work best. Understanding how genetic mutations cause or contribute to disease also gives scientists and physicians powerful tools to screen people who may be at risk.
As we learn more about Alzheimer’s, Parkinson’s and Lewy body dementia and the role of genetic factors, it is becoming increasingly clear that a complex and varied set of influences impact the chances of disease onset. The more we know about each of these components will bring us one step closer to urgently needed treatments that improve quality of life for people around the world.

 ALZHEIMER’S DISEASE
ALZHEIMER’S DISEASE