GRAND RAPIDS, Mich. (June 22, 2023) — Loss of two key “protector” proteins initiates epigenetic changes that transform healthy lung cells into cancerous ones, according to new research from Van Andel Institute scientists.

The findings, published in Cancer Research, are among the first to demonstrate a wholly epigenetic origin for cancer cells and may have implications for future cancer treatment and prevention strategies.
“Our findings shed new light on the importance of epigenetics in cancer development,” said Gerd Pfeifer, Ph.D., a VAI professor and senior study author. “In theory, it is easier to target epigenetics than genetics in cancer treatment, which opens new possibilities for therapeutic development.”
Cancers largely arise from mutations to the DNA sequence, which disrupts the genetic instructions required for normal function. The resulting errors allow malignant cells to flourish and spread, crowding out healthy cells and causing illness.
Since the 1980s, however, scientists have recognized the role of another critical regulatory system in cancer development: epigenetics.
Epigenetic mechanisms govern whether genes are “on” or “off” by adding or removing chemical tags called methyl groups. Inappropriate methylation wreaks havoc by activating or silencing genes at the wrong time; for example, a gene that regulates cell death may be erroneously switched off, allowing cancerous cells to replicate unchecked.
Although epigenetic mechanisms are now recognized as central players in cancer, exactly how these processes work has remained unclear.
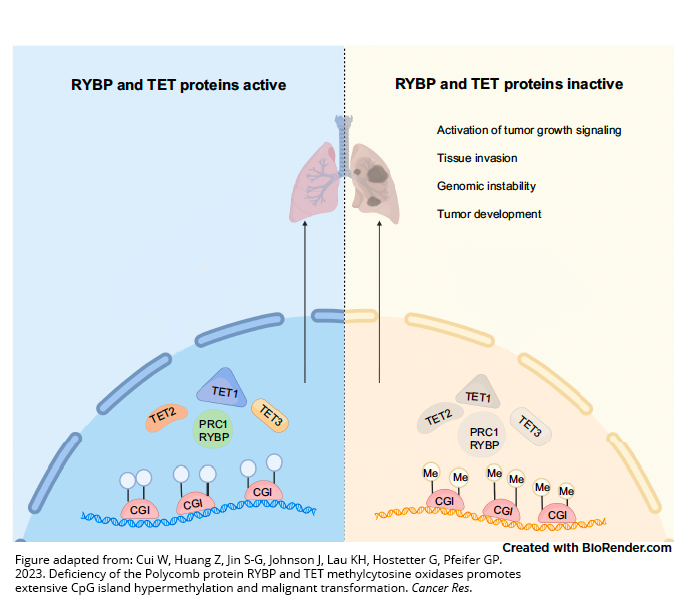
To find answers, Pfeifer and his colleagues focused on two proteins that protect more than 4,000 genes from inappropriate methylation: TET and RYBP.
Their research, which was conducted in lab models of lung cancer, revealed that loss of either TET or RYBP on their own has only minimal to
moderate impact. Loss of both, however, leads to widespread aberrant methylation — and, subsequently, cancer.
Their findings suggest the combination of RYBP and TET is crucial to preserving normal function. RYBP helps maintain a massive protein complex called PRC1, which shields thousands of genes from methylation. TET proteins protect the genome by removing methylation when it is not appropriate. When these protective mechanisms break down in tandem, methylation spins out of control.
Going forward, Pfeifer plans to explore this process in other cancer types to investigate whether it’s a phenomenon restricted to lung cells or if it has wider applicability. If the combined loss of RYBP and TET in other cell types has the same result, it could have broad implications for our understanding of cancer and development of new therapies.
Authors include Wei Cui, Ph.D., Zhijun Huang, Ph.D., Seung-Gi Jin, Ph.D., Jennifer Johnson, M.S., Kin H. Lau, Ph.D., and Galen Hostetter, M.D., of VAI. VAI’s Genomics Core, Pathology and Biorepository Core, Optical Imaging Core and Vivarium Core also contributed to this work.
Research reported in this publication was supported by Van Andel Institute and the National Cancer Institute of the National Institutes of Health under award no. R01CA234595 (Pfeifer). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Banner image credit: Courtesy of Adobe Stock — catalin.